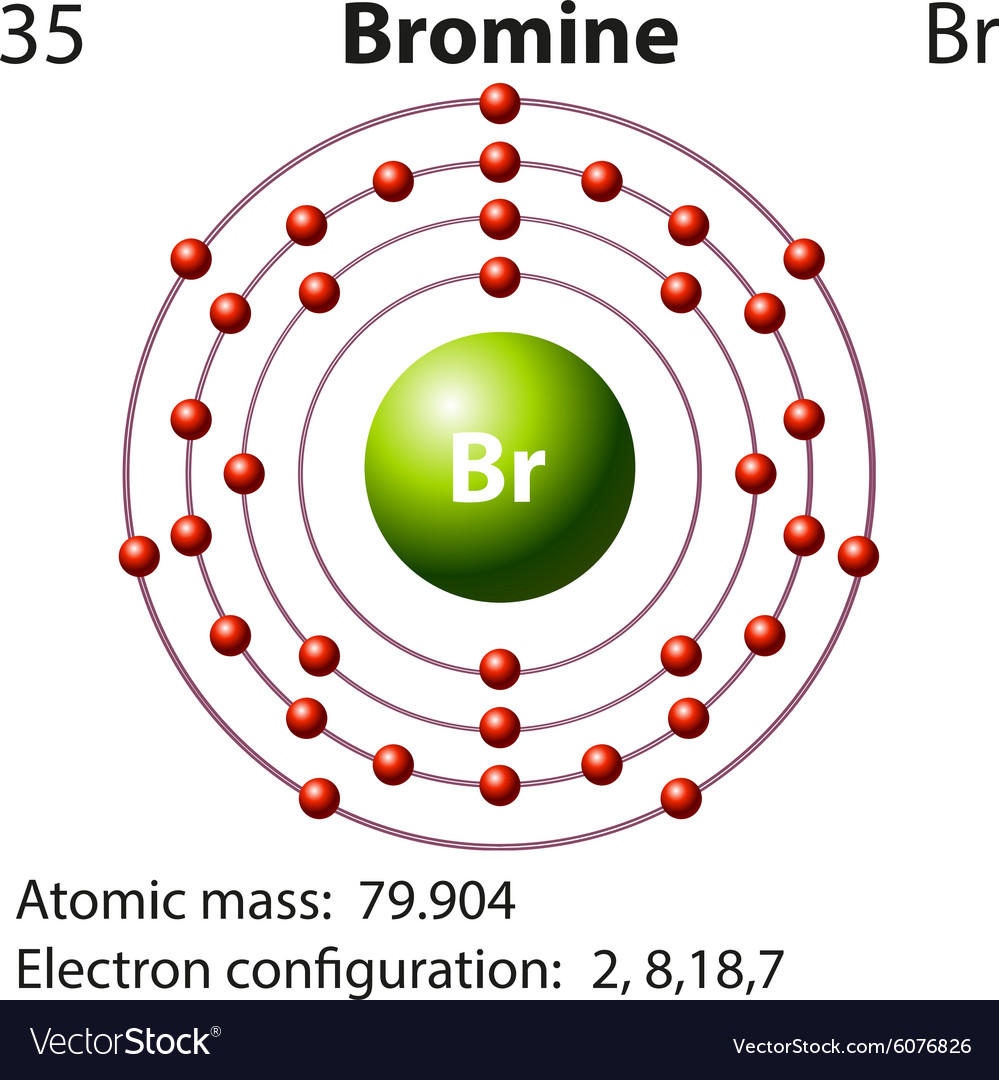
Does Bromine Lose Electrons . For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 17 gain one electron and form anions with a 1− charge; Learn for free about math, art, computer programming, economics, physics, chemistry,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. ) and reduction (gain of electrons). Atoms of group 16 gain two electrons and form ions with a 2− charge,. Redox reactions involve both oxidation (loss of. It was the first element to be extracted.
from www.vectorstock.com
) and reduction (gain of electrons). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Learn for free about math, art, computer programming, economics, physics, chemistry,. Redox reactions involve both oxidation (loss of. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of group 17 gain one electron and form anions with a 1− charge; It was the first element to be extracted.
Symbol and electron diagram for Bromine Royalty Free Vector
Does Bromine Lose Electrons It was the first element to be extracted. It was the first element to be extracted. Atoms of group 17 gain one electron and form anions with a 1− charge; Learn for free about math, art, computer programming, economics, physics, chemistry,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. ) and reduction (gain of electrons). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Redox reactions involve both oxidation (loss of.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Does Bromine Lose Electrons For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 16 gain two electrons and form ions with a 2− charge,. It was the first element to be extracted. ) and reduction (gain of electrons). Redox reactions involve both oxidation (loss of. Atoms that lose. Does Bromine Lose Electrons.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Does Bromine Lose Electrons Learn for free about math, art, computer programming, economics, physics, chemistry,. ) and reduction (gain of electrons). Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Atoms of group 17 gain one. Does Bromine Lose Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Does Bromine Lose Electrons Learn for free about math, art, computer programming, economics, physics, chemistry,. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 17 gain one electron and form anions with a 1− charge; Redox reactions involve both oxidation (loss of. Atoms of group 16 gain two. Does Bromine Lose Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Does Bromine Lose Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Learn for free about math, art, computer programming, economics, physics, chemistry,. ) and reduction (gain of electrons). Atoms of group 17 gain one electron and form anions with a 1− charge; It was the first element to. Does Bromine Lose Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Does Bromine Lose Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Learn for free about math, art, computer programming, economics, physics, chemistry,. Redox reactions involve both oxidation (loss of. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Does Bromine Lose Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Does Bromine Lose Electrons Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. It was the first element to be extracted. Atoms of group 16 gain two electrons and form ions with a 2− charge,. For example, the neutral bromine atom, with 35. Does Bromine Lose Electrons.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Does Bromine Lose Electrons It was the first element to be extracted. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Learn for free about math, art, computer programming, economics, physics, chemistry,. Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because. Does Bromine Lose Electrons.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Does Bromine Lose Electrons It was the first element to be extracted. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 17 gain one electron. Does Bromine Lose Electrons.
From www.numerade.com
SOLVED 0 / 1 pts Question 11 As per octet rule, bromine atom would Does Bromine Lose Electrons It was the first element to be extracted. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms that. Does Bromine Lose Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Does Bromine Lose Electrons ) and reduction (gain of electrons). Learn for free about math, art, computer programming, economics, physics, chemistry,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with. Does Bromine Lose Electrons.
From robertanderson.z13.web.core.windows.net
Does Bromine Gain Or Lose Electrons Does Bromine Lose Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Redox reactions involve both oxidation (loss of. It was the first element to be extracted. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.. Does Bromine Lose Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Does Bromine Lose Electrons Redox reactions involve both oxidation (loss of. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Learn for free about math, art, computer programming, economics, physics, chemistry,. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons. Does Bromine Lose Electrons.
From studylib.net
By GAINING or LOSING electrons Does Bromine Lose Electrons Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. ) and reduction (gain of electrons). For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. It was. Does Bromine Lose Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Does Bromine Lose Electrons For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer. Does Bromine Lose Electrons.
From www.vectorstock.com
Symbol and electron diagram for Bromine Royalty Free Vector Does Bromine Lose Electrons It was the first element to be extracted. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. ) and reduction (gain of electrons). Atoms of group 17 gain one electron and form anions with a 1− charge; Learn for free about math, art, computer programming, economics,. Does Bromine Lose Electrons.
From library.achievingthedream.org
Periodic Variations in Element Properties Introductory Chemistry Does Bromine Lose Electrons Atoms of group 17 gain one electron and form anions with a 1− charge; Learn for free about math, art, computer programming, economics, physics, chemistry,. Redox reactions involve both oxidation (loss of. ) and reduction (gain of electrons). Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance. Does Bromine Lose Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Does Bromine Lose Electrons Learn for free about math, art, computer programming, economics, physics, chemistry,. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons. Does Bromine Lose Electrons.
From www.youtube.com
Which elements tend to lose electrons? What charge will they Does Bromine Lose Electrons Atoms of group 16 gain two electrons and form ions with a 2− charge,. Redox reactions involve both oxidation (loss of. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. It was the first element to be extracted. Atoms of group 17 gain one electron and. Does Bromine Lose Electrons.